When a serious accident occurs in a nuclear power plant, a large number of radioactive fission products are released from the core due to meltdown, which will cause environmental pollution and casualties. The amount of core source terms directly affects the quantity released. Jia analyzed the important actinides nuclides, fission product nuclides, and activation product nuclides and proposed a reasonable disposal plan. Liu and Zhu proposed an analysis model of the minimum nuclear criticality accident source term and provided a related calculation method. Wheeler et al. used Serpent 2 to calculate the fuel consumption of molten salt reactors and found that gas removal can affect many different fission products beyond the gaseous chemical group. Actinides were also affected, although not in any gaseous fission product decay chain.
Sun et al. used MELCOR to study the source term estimation of an AP1000 nuclear power plant under severe accident conditions. Liu et al. conducted a series of experiments on the 14C source term of a 10 MW high-temperature gas-cooled reactor. Fang et al. carried out ab initio calculations of the antineutrino flux of a new isotope reactor and provided accurate numerical calculations of the lepton wave function.
How To Calculate Reactor Jacket Volume Liu et al. analyzed the problems in the calculation of the fission product source term of M310/CPR1000, EPR, and AP1000 and modified the calculation process of the fission product source term. The analysis results showed that the decontamination factor for nuclide groups, excluding inert gas, Sb, and Te2 nuclide groups, was approximately 2.5. Bahadir and Lindahl used the nodal code SIMULATE to simulate the reactor core to calculate the node-wise burnup and the power of the target assembly under different burnups. They found that SIMULATE-5 can accurately describe the neutronic and thermal-hydraulic behavior of boiler water reactor and pressured water reactor cores. Gera et al. estimated the source terms of some postulated severe accident scenarios in the 220 MW Indian pressurized heavy-water reactor. They found that the estimated source term and corresponding consequences were higher in reactor inlet header break cases than in reactor outlet header break cases.
Ahn et al. used MELCOR 2.2 and MAAP 5.04 to calculate the source terms of a PWR after a serious accident. They found that the dedicated mitigation strategy greatly decreased the environmental release of the fission product cesium. With regard to the evolution of severe accident and plant responses, both codes predicted the general trend of each base and mitigation scenarios. There are relatively few studies on the calculation of the weight of factors affecting the core source term in this type of literature.
By calculating the radioactivity and photon source strength of actinides and fission products, the weight proportion of each factor was calculated by the multifactor variance analysis method . In this study, the key parameters for the calculation of the source terms for supercritical water-cooled reactors were selected. The ORIGEN2 code was used to simulate the radioactive activity and photon source strength under different fuel consumptions, enrichments, specific powers, and operation modes.
The results show that the radioactivity of actinides and fission products increased with the increase in fuel consumption and decreased with the increase in enrichment. The radioactivity of fission products and actinides varied linearly with the specific power, with a correlation coefficient of 1. Simultaneously, a multifactor analysis program was established to calculate the influence of various factors on the activity of fission products. It was found that the specific power was the most important factor, followed by the enrichment degree; fuel consumption was the least important.
The calculation of the core source term is affected by various factors, such as fuel consumption, enrichment, specific power, and operation mode. The activity of lanthanides, fission products, and the photon source strength were calculated using the ORIGEN program. The weights of each factor were calculated by multivariate analysis of variance. The results show that the radioactivity of actinides and fission products increased with the increase in fuel consumption.
As enrichment increased, the radioactivity of fission products and actinides decreased. The radioactivity of fission products and actinides increased linearly with the change in specific power, with a correlation coefficient of 1. The changes in fuel consumption and enrichment have little effect on low-energy photons, but significantly affected high-energy photons.
The change in specific power has little effect on the photon generation of different energy groups. The operation mode has little effect on the radioactivity of the nucleus and fission products. Multivariate analysis of variance shows that specific power is the most influential factor, followed by enrichment; the least influential factor is fuel consumption. Retinol was the first product that was produced on industrial scale with Microreactor Technology at the our facility in Buchs.
The batch synthesis procedure that was used before the transfer into a flow chemistry process was unreliable and low yielding. The underlying chemical conversion - a simple basic ester hydrolysis - is not the cause of problems in this case . The ester is cleaved quantitatively, but this process takes some time until it is completely finished. Portions of cleaved product that are formed right at the beginning of the process need to stay in the batch reactor under reaction conditions until all the remaining ester is cleaved. During this time the majority of the highly sensitive product degrades.
Another observation was a serious unreliability of the batch process. Due to small and uncontrollable contributing factors, the yield of the reaction varied tremendously. In the continuous flow, every portion of product is streamed out of the reaction zone instantly after it has formed. The product outlet can lead directly into a protected storage vessel where the sensitive product is protected from degradation.
The flow process facilitates quantitative conversion where as any yield lost is during work-up. It should be highlighted that the savings achieved with this single new synthesis procedure have already justified all our investments into microreactor technology. Today, microstructured devices offer greatly enhanced mixing and heating capabilities compared to the batch process, leading to improved product profiles and higher yields. Thus, microreactors might be regarded as the chemist's round-bottomed flask of the 21st century . With a reactor volume of less than a milliliter, flow chemistry allows the scale-independent synthesis from g to kg amounts in a single day.
The small reactor volume facilitates the safe and easy handling of hazardous or instable materials and highly exothermic reactions. Fast and easy parameter screening makes Microreactor Technology an ideal tool for process development. The conversion of feed to products is the essence of a chemical process and, thus, the reactor is the heart of a chemical plant. When designing a reactor, an engineer must first collect data about the chemical reaction and then select appropriate reaction conditions, which will help determine suitable materials of construction. Next, the designer should determine the rate-limiting step and, from this, the critical sizing parameter.
Next, preliminary sizing, layout, and costing can be conducted for the reactor. At this point, simulations and experiments can be conducted to verify that the proposed reactor will meet the desired specifications. Space velocity relate the flow rate of a gas or liquid feeding a reactor, divided by the amount of of catalyst that is going to be placed in the reactor.
Space velocity is not the same that inverse of residence time, it needs to have specific condition for that..... The residence time unit on the glass microreactor is fixed to 0.66 mL. Reactions requiring a longer residence time variable, extension is possible with 25 mL of extra PTFE tubing supplied with the kit. The residence time τ is the flow chemistry equivalent of the reaction time in classical batch chemistry. It is defined by the combined volume of the microreactor and the residence time unit and the flow rate. The PTFE tubing can be cut down exactly to the length needed for a specific reaction.
Smaller pieces can be combined again into longer residence time units with connectors. To control the reaction temperature the tubing can be coiled and submerged in a heated or cooled bath. Optimum size is achieved when the area above the rectangles from the CSTRs in series that was previously covered by a single CSTR is maximized. For a first order reaction with 2 CSTRs, equal volumes should be used. As the number of ideal CSTRs approaches infinity, the total reactor volume approaches that of an ideal PFR for the same reaction and fractional conversion. In sharp contrast to the batch mode, chemical synthesis becomes a time-resolved process in flow chemistry.
Reagent streams are continuously pumped into a flow reactor where they are mixed and allowed to react. Therefore, only flow rate and operation time determine the synthesis scale. Per day control is probably the most simplistic method by which a perfusion process can be developed and operated. Typically, a flow rate is set per day and increased as the cell concentration increases.
This can be achieved using a commercially available CD-CHO type growth media, typically starting at 1 RVD once any lag phase has been passed, and the cells start growing exponentially. The RVD is increased daily based on the observed growth rate and basic metabolomics data such as bioreactor glucose, lactate, and ammonia concentrations. Depending on the cell line and the final cell density required, the final RVD can increase to between 5 and 10 RVD.
The general rule is if the growth rate slows, then the RVD is increased. In this way, depletion of key metabolites and/or build-up of growth inhibiting byproduct can be balanced. This is a very simple way of starting a perfusion process development.
However, the resulting process will likely consume significantly more volume of media (measured as a cell specific perfusion rate pL/cell/day) than a well-developed process. The weight of each impression factor was calculated and analyzed by multivariate analysis of variance. Based on the data in Table 2, an orthogonal table of fuel consumption, specific power, and enrichment was established, as shown in Table 3. The borosilicate glass microreactor chip manufactured by Little Things Factory in Germany following our design, offers the highest possible resistance to aggressive reagents . Even corrosive reactants like concentrated acids or acid halides can be used without harm. Glass offers the obvious advantage, where processes taking place in the microreactor are clearly visible.
Blockages and impurities are easily detected and their removal can be followed visually. At the same time, channel dimensions are small enough to benefit from all advantages of microreactor technology . A seperate channel allows insertion of the temperature sensor supplied with the kit directly into the glass chip. The temperature can be measured freely at any location between the beginning of the mixing zone to the end of the residence time unit. This becomes even more evident when looking at the scaling-up of a production routine.
A synthesis procedure that works well in a small glass flask in R&D may pose huge problems when transferred directly to larger vessels in a kilo lab or pilot plant. Time consuming process development is necessary to fit the synthesis to the different parameters of a bigger reactor vessel. In flow chemistry a single microreactor can cover a broad range of production scales from mg to kg. For scaling-up, only the operation time of the system is extended.
As an alternative to an enzyme catalyst, engineered microorganisms can be used to produce a chemical of interest. This product could be complex biological compounds, therapeutic proteins, or commodity plastics and fuels . Host cells as a platform for modification have so far included bacteria, yeast, and mammalian cells . The efficiency of a bioreactor is heavily dependent on the efficiency of the microorganism used. An inefficient cell host that does a poor job of producing the desired product will always result in a poorly designed bioreactor, regardless of the equipment or conditions used.
Furthermore, the design of a bioreactor is largely based around the ideal growth conditions of the microorganism. As shown in this section, the design and/or selection of a microbial host is closely related to the design of the bioreactor. This process can involve the rigorous engineering of a novel microorganism, a large screening for high producing strains, or, most likely, a combination of the two. This step of the bioreactor design process requires close collaboration between process engineering and microbiology.
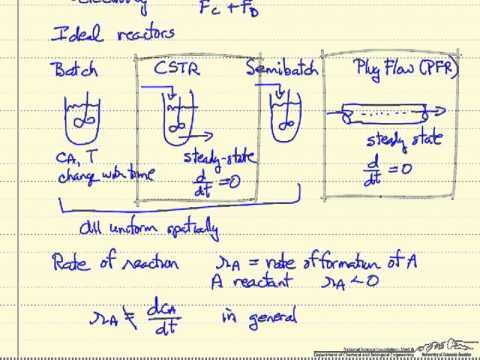
The behavior of a CSTR is often approximated or modeled by that of an ideal CSTR, which assumes perfect mixing. In a perfectly mixed reactor, reagent is instantaneously and uniformly mixed throughout the reactor upon entry. Consequently, the output composition is identical to composition of the material inside the reactor, which is a function of residence time and reaction rate.
The CSTR is the ideal limit of complete mixing in reactor design, which is the complete opposite of a plug flow reactor . In practice, no reactors behave ideally but instead fall somewhere in between the mixing limits of an ideal CSTR and PFR. The rotary piston pumps provided with kit represent a very robust system with appropriate specifications for continuous flow chemistry.
Metabolic engineering studies ways to relieve these stresses by "rewiring" synthesis networks within cells . The first involves constructing non-native biochemical pathways in cells. This is necessary if the host does not already produce the desired product. Enzymes are expressed in the host that catalyze the correct reactions to synthesize the product. This often puts stress on cells, as it diverts resources in the cell towards the product that are typically utilized elsewhere, such as for growth.
The second aspect of metabolic engineering involves the manipulation and balancing of metabolic fluxes within the cell. This involves controlling the expression of enzymes so that the cell makes enough product, but still has enough resources to grow to an acceptable level. Sometimes, it can be advantageous to only induce production of the product after cells have grown to a high concentration. This requires the heterologous DNA to be expressed with an inducible promoter.
For example, production of a product could be induced when the feedstock is switched to methanol . Reactions are usually carried out in liquid or gas phases as fluids are easier to handle, heat and cool, and transport than solids. For reagents or products in the solid phase a suspension in liquid or gas is usually used.
The phase of the reaction is usually determined by reactor temperature and pressure. Liquid-phase operation is usually preferred due to the highest concentrations and greatest compactness. However, at temperatures above the critical temperature there cannot be a liquid phase. The pressure can sometimes be adjusted to keep all reagents in the liquid phase, however when this is not possible a multiphase reactor will be necessary.
















No comments:
Post a Comment
Note: Only a member of this blog may post a comment.